Discover the integration power of SAP Quality Management vendor release and what you need to set up to trigger the functionality.
Key Concept
The vendor release date is usually set either far into the future (which means when it’s released, it’s permanent) or it is an actual vendor/material review date. If it is entered as a review date, then you must monitor the dates to see if the review is due to be performed. The actual review steps vary by company but most have a procedure in place for conducting a vendor/material review, such as a site visit or review history. In both cases, entering the release date signifies the vendor/material review is complete and purchasing can begin buying.
With SAP Quality Management (SAP QM) vendor release, purchasing can clearly see whether or not a vendor/material combination has been released in the purchase order create screen because an error message appears if it has not been released. Once the error message appears, purchasing can then contact the quality department to let them know that the release needs to be performed. However, when SAP QM vendor release is implemented, it is important to have the internal procedures already in place to ensure vendor/material releases are done ahead of time. This way, purchasing can avoid the error messages altogether.
In my opinion, the SAP QM vendor release functionality is not being implemented as often as it should. One reason is that many clients are not aware the functionality is available. A number of clients I’ve spoken with said they would have implemented this functionality had they known it was available. You will see in this article how powerful SAP QM vendor release is and how it is one of the easier parts of QM to implement. The SAP QM vendor release functionality allows the quality department to control the release of vendors when there is a new product, revision, or change of vendor. The SAP QM vendor release functionality ensures that premature buying (i.e., buying before samples have been approved by the quality assurance (QA) department for initial buy) is not possible and gives the QA department the chance to review and release the vendor/material combination for purchase.
In most business environments, there is a policy or procedure in place stating that before purchasing can begin buying a new product or change vendors, the quality department needs to approve the vendor/material combination or, in the case of a new vendor, approve all the materials (e.g., by samples or site visits) that the vendor will be supplying. Even though this is written down, many times the process is not followed. By implementing the SAP QM vendor release functionality with the error setting turned on in configuration, purchasing cannot buy the material until the QA department has released the vendor/material combination. If someone tries to purchase a material without the QM release, the system sends a hard error message. With this functionality in place, the QA department can perform its due diligence review to ensure the vendor can meet the product requirements and specifications on a buy-to-buy basis. If this is the policy within your organization (which is most often the case), then no one should oppose turning on this functionality.
Note
SAP QM vendor release is available on all SAP versions.
Figure 1 shows the error message that purchasing receives after trying to purchase a material that has not yet been released by the quality department. The following section details how to activate the vendor release setting in the material master QM view and how to configure the error message functionality.

Figure 1
Error message due to a lack of a QM vendor/material release
Turning on SAP QM Vendor Release Functionality
The following three steps show the master data and key configuration needed for turning on the SAP QM vendor release functionality:
Step 1. Material Master SAP QM Vendor Release Setting
Use transactions MM01 or MM02 on the material master QM view to activate the SAP QM vendor release functionality. First you check the QM proc. active box. Next, you enter the QM Control Key (
Figure 2) that has the vendor release setting turned on (see
Figure 3 for the release required setting). After checking these two fields, the material cannot be purchased without the quality department releasing the product.

Figure 2
Material master QM release triggers
Step 2. Configuration — QM Control Key
There are two main settings you must make in the QM control key configuration. The first is the Release required check box. This setting turns on the quality vendor/material release requirement. Next is the Message mode, which you should set to E Defects (
Figure 3). This setting creates the purchase order error if the QM release is not entered in the system.

Figure 3
Vendor release control key configuration
Step 3 consists of three parts:
- Vendor release with release date
- Vendor release with quantity limit
- Vendor release using the mass release screen
Vendor Release with Release Date
When activating the SAP QM vendor release functionality in the material master, use transaction QI01 to create an entry in the quality information (Q-info) record for the vendor/material combination before purchasing can begin to purchase the combination. Once in the Q-info record (
Figure 4), you are required to enter the Material, Vendor, and Plant on the entry screen and then the Release Until date inside the detail screen. The Release Until date most often used is a future date, such as 01/01/2999. However, in some cases companies use the Release Until date as the next vendor/material review date. Once the review is done, the date is moved out for the next review. The Release Until date has a dual purpose in this case. You can also use program QM Release Mass Maintenance to monitor what vendor/material reviews are coming up. I discuss this in more detail in the Q-Info Record – Mass Vendor/Material Releasing section.
Note
Be sure that when you use the Q-info record functionality, you perform all the approval processes for releasing a vendor/material in your internal policy and procedures. Do not fall into the trap of weakening this functionality by creating the Q-info record without the approval.
Note
This article focuses on one trigger inside the Q-info record. Other triggers within Q-info record, such as first article and vendor inspect override, are beyond the scope of this article.
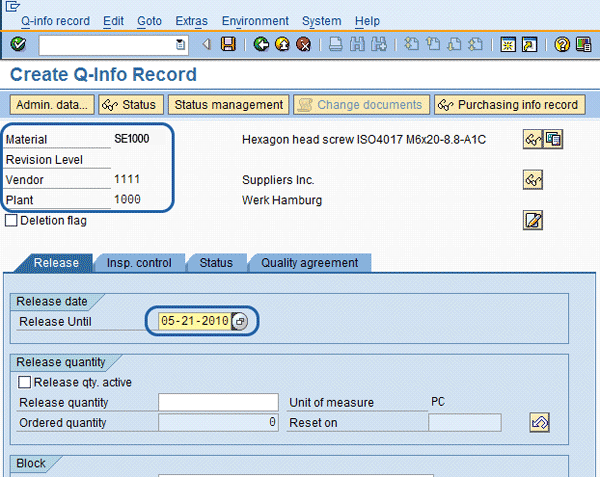
Figure 4
Q-info record for the vendor release
Q-Info Record — Vendor Release with Quantity Limit
The Q-info record not only allows the Release Until date, it also allows a Release quantity (
Figure 5) to be purchased before you open it up to full quantity purchases. This allows the quality department to approve and release a small amount of the materials for purchase and use it in a production sample before buying a large quantity and possibly having it fail. Once the initial sample is approved, the Release qty. active box is unchecked and the Release Until date is used.

Figure 5
Vendor release using purchase quantity limit
Now that the Q-info record is created for the vendor/material being purchased, the purchase order goes through without any errors (
Figure 6).

Figure 6
No error messages because the vendor/material are released
Q-Info Record — Mass Vendor/Material Releasing
The Q-info release mass maintenance program is used for a number of functions (
Figures 7 and
8). This screen is another way to do the vendor/material release, but it does so in a mass mode for multiple vendor/material combinations. It is typically used at the start of turning on the SAP QM Vendor Release functionality because it looks at all purchasing info records that do not have a matching Q-info record and shows them to the screen to allow initial mass release. For each purchasing info record, it allows you to create a Q-info record and enter the date for the Release Until. After the initial creation of the Q-info records, this program is used to monitor any new purchasing info records that do not have a Q-info record. This ensures the approval process is not getting behind and the correct process is being followed. Additionally, if the Release Until Date is also being used for the next vendor/material approval date, then this program is used to keep track of upcoming reviews.
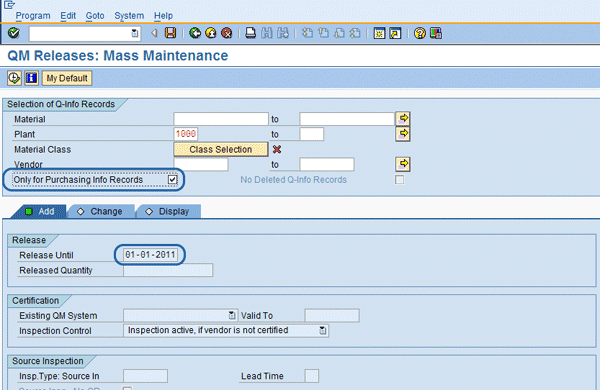
Figure 7
Q-info record mass vendor/material releasing
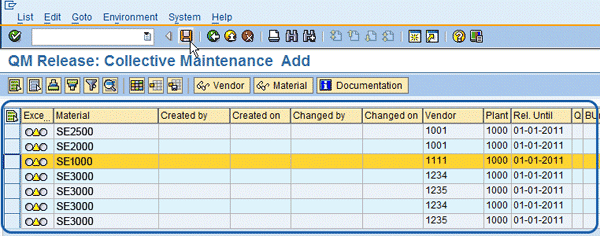
Figure 8
Q-info record – mass vendor/material releasing
Doug TenBrock Sr.
Doug TenBrock is a senior QM/MM consultant with Enowa with 15-plus years of SAP project experience. His experience includes 17 full life cycle projects, project management, and the integration of all SAP modules. Doug’s industry exposure includes chemicals, pharmaceuticals, food, bio tech, health care, and high tech. The knowledge that he has gained from the diversity of the projects worked on has helped him create tools for streamlining system development, effective training of users, and assistance to colleagues in system problem solving. Doug is a team player who ensures that all modules successfully cross the finish line together.
You may contact the author at
tenbrockd@msn.com.
If you have comments about this article or publication, or would like to submit an article idea, please contact the
editor.